People’s interest to know about the smallest particles of the matter was increasing day by day. If you cut a brick into two pieces, you get two bricks. Even if it is divided into two pieces, it is obtained. If you break it further, you will get bricks. In this way, you can cut any object into an infinite number of pieces, when it can no longer be cut into pieces. What will this smallest particle of matter look like in this situation? How will his behavior be? Is it possible to break it down in any other way? If you can break it, what can be found from there? These questions make people think. People want to know. As there were various fairy tales or stories of people about space, there were no such stories about this smallest particle of matter. Because not everyone thought much about it except science-minded people.
One of the people who thought about this smallest particle of matter was an ordinary school teacher. His name is John Dalton. He mentions this – If we cut any object into small pieces, if we take it to a state, which can no longer be cut, that is the smallest particle of matter. It is completely indivisible. Later Democritus gave the name atom or atom. That this smallest particle of matter is indivisible was accepted by many eminent scientists at that time. There was another group that said that atoms are not indivisible. Rather it is divisible. And if it is broken, its constituent elements will be found.
Thomson’s Atomic Model
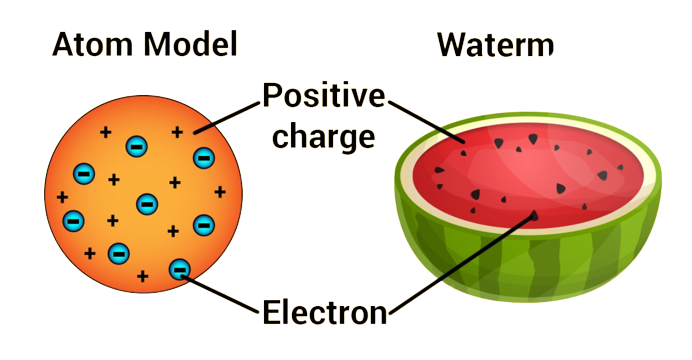
Then in 1897, Joseph John Thomson discovered electrons from cathode rays, which are negatively charged. He further established the idea that atoms are fissionable. In 1904 he gave the atomic model which we know as Watermelon or Plum Pudding model. He compared the atom to a watermelon. He pointed out that the atom as a whole is like a watermelon, where the whole watermelon is an atom with a continuous positive charge. In this, the electrons are bound according to the electrical interaction. That is, watermelon seeds can be thought of as one electron. Where the hydrogen atom looks like a speck. Since the nucleus, proton or neutron had not been discovered till then, there is no idea about them in his model.
The biggest limitation of Thomson’s atomic model was the construction of positive and negative charges. He was able to make a strong argument that atoms are divisible despite limitations.
Rutherford’s Atomic Model
Kenneth Robert Rutherford experimented in 1911 with the help of his two assistants Geiger and Marsden to make the idea that atoms are divisible and what is inside them a reality. He directed alpha rays between very thin gold sheets and used a screen of zinc sulfite on the opposite side. So that the scattered alpha particles pass through the atoms and fall onto the screen. And from the number and behavior of alpha particles that come out, he can give an idea about the internal structure of the atom.
He found that most of the alpha particles left the source and passed through the atoms straight into the screen. But the particles which are moving past the center of the atom, they are bending. And those who passed through the center were returning. However, the number of these returned or bent particles was negligible. He points out that there is definitely a very strong and heavy object in the center of the atom due to which the strong alpha particles are bent or bounced back. Again, since most of the space in the atom is empty, we understand the mass of this heavy center as the mass of the atom. He named it its central nucleus.
Since the entire atom is overall charge neutral, there are as many negatively charged electrons around the atom as there are positively charged protons in the center of the atom. Earlier in 1909, Rutherford also discovered positively charged protons in foil experiments.
He mentions that just as the planets of the solar system revolve around the sun, the electrons in atoms revolve around the nucleus. The centrifugal force acting due to the spin of these spinning electrons and the static electrostatic force of attraction or Coulomb force between the electron and the nucleus are equal to each other.
Although his model had many limitations, he was able to answer almost all questions about atomic fission. Also, his discovery of the nucleus and his proton in 1909 was one of the milestones in the history of atoms.
Limitations of Rutherford’s Atomic Model
Although Rutherford’s atomic model had many successes, it also had many limitations. His main limitation was comparing the rotation of electrons around the nucleus of an atom with the rotation of the planets around the sun in the solar system. Both planets and the sun are charge neutral in the solar system. On the other hand, electrons and nuclei are charged particles.
A question may come to the mind of many – the charge may be added, but the method of rotation is the same. But a charged object orbiting another oppositely charged object and an uncharged object orbiting another uncharged object are not the same. Maxwell showed that if a charged object revolves around another charged object, it will continuously radiate energy and eventually fall to the center. As it happens in the atom, the destruction of the atom is sure. But no such breakdown occurs in atoms. So from this point of view, Rutherford was not able to give stability to the atom. Again, Johannes Kepler showed that the planets of the solar system do not revolve around the sun in circular but elliptical orbits. In that case, Rutherford did not give any idea about the orbit of the electron.
Again, Rutherford did not give any idea about the spectrum of atoms. Which carries a lot of important information for an atom. Even by looking at the atomic spectrum, we can determine the identity of each atom.
Bohr Atomic Model
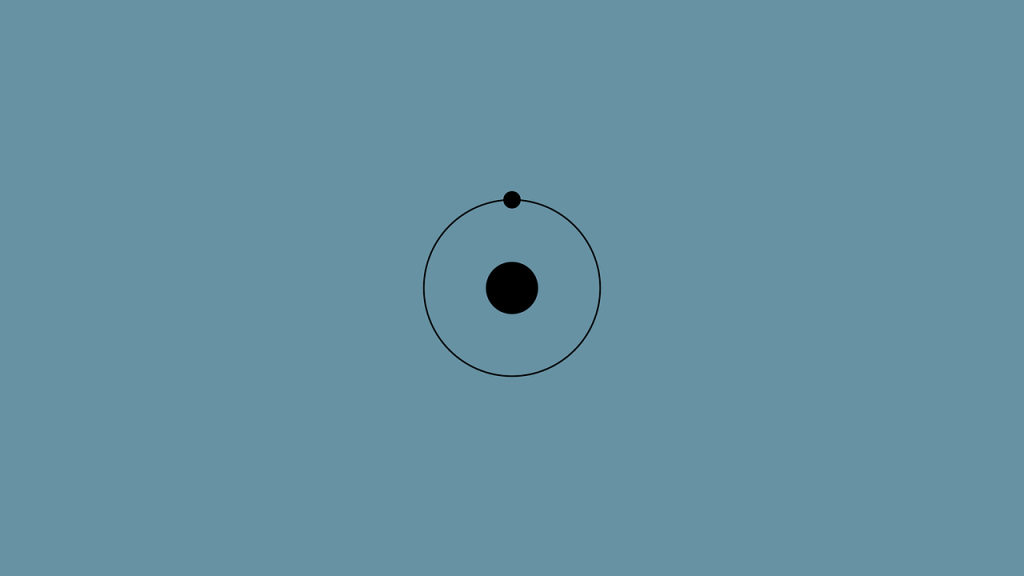
Rising from Rutherford’s limitations, scientist Niels Heinrich David Bohr published his model of the atom in 1913. In his model he mentions-
Electrons travel in specific circular orbits around the nucleus. Where the centripetal force of the electron due to rotation and the static attraction force or Coulom force of the electron with the nucleus is equal to each other.
Momentum is a very important quantity for any moving object, whether circular, straight line, or otherwise. He determined the value of the angular momentum of the rotating electron in his model. From the mathematical form of the angular momentum of the electron and the centripetal and centrifugal force, we can easily determine the energy of the electron in any orbit of the atom, the velocity of the electron, and the radius of the orbit or the distance of the electron from the nucleus. Which was our big achievement for the atom.
Bohr showed that an electron has a certain amount of energy when it is in any orbit. That energy value is lower for lower energy levels and higher for successively higher energy levels. When an electron moves from a lower energy level to a higher energy level, the electron absorbs energy to gain additional energy. Again, when the electron from the highest energy level comes to the lower energy level, it releases excess energy or radiates. Regardless of whether this absorption or radiation occurs, energy absorption and transfer of electrons during radiation form the absorption and radiation spectra respectively. Where Bohr gave a clear idea about the atomic spectrum.
Here, the spectrum formed when an electron transitions from the highest energy level to the first energy level is called the Lyman series. Similarly, the electron transition from the highest energy level to the second energy level is called the Balmer series. The transition of electrons from the highest energy level to the third energy level is called the Paschen series. The transition of electrons from the highest energy level to the fourth energy level is called bracket series. Electron transition from the highest energy level to the fifth energy level is called fund series. And when the electron transitions from the highest energy level to the sixth energy level, it is called the Humphreys series.
Among the many successes of Bohr’s atomic model is the determination of electron momentum, energy, velocity, and orbital radius. Determine the shape of the electron orbit. Concept of atomic stability and spectrum. However, this model also had significant limitations.
Limitations of the Bohr Atomic Model
Bohr’s atomic model and its calculations are all applicable to hydrogen, an atom with one proton and one electron. It is not acceptable for all other atoms.
According to the concept of Bohr’s spectrum, a spectrum or spot is supposed to be created between the two orbitals as a result of the electron moving to the highest energy level or arriving at the lower energy level. But apart from this spectrum in atoms, we see many small spectral lines next to it. Bohr could not give us a clear idea of the cause and origin of these.
Even so many models about the atom could not end the speculations about the atom in the minds of people. One question after another about the atom kept arising in their mind. One such thinker was James Chadwick. He discovered the existence of an uncharged but intrinsically magnetized particle with protons in the nucleus of the atom. Named Neutron.
By that time, people have found answers to many questions about the smallest particles of matter, electrons, protons, and neutrons. But there were some limitations hidden in everything. Just as with the latest Bohr atomic model, restrictions are introduced only for atoms other than hydrogen atoms. So we need a model that answers all our questions about atoms. But how?
We continue to discover and gain knowledge about atoms and particles of small mass and size. Among these, Heisenberg’s Uncertainty Principle, De-Broglie’s Particle-Wave Duality, Max Planck’s Photon Concept, Schrödinger’s Electronic Equation, etc. were notable. And based on these theories, we get the quantum model in the joint edition of Max Planck and Albert Einstein.
Since the discovery of the quantum model or quantum mechanics, new information about the atom began to come into our hands. We learn many limitations and new information about Rutherford and Bohr’s ideas about the atom. Notable among which was Paul Dirac’s concept of the superposition of electrons. Whereas electrons do not exist in all places simultaneously. The electron has broken the possibility-impossibility barrier here.
The divisibility of atoms is fully proven today. Where by breaking atoms we get electrons, protons, and neutrons. Now the modern quantum model also tells us – these electrons, protons, and neutrons are also divisible. They are made up of many more tiny particles. One day it may be discovered that all those particles also come from some energy. This may be the secret of quantum energy or vacuum energy and quantum fluctuations.
Many still believe that we will soon discover many more unknown facts about atoms from the quantum model. So quantum mechanics has a separate place in the minds of physics lovers.
Writer
Jeion Ahmed
Chittagong, Bangladesh

Leave a comment